Brachial plexus injury Part II: the road to recovery

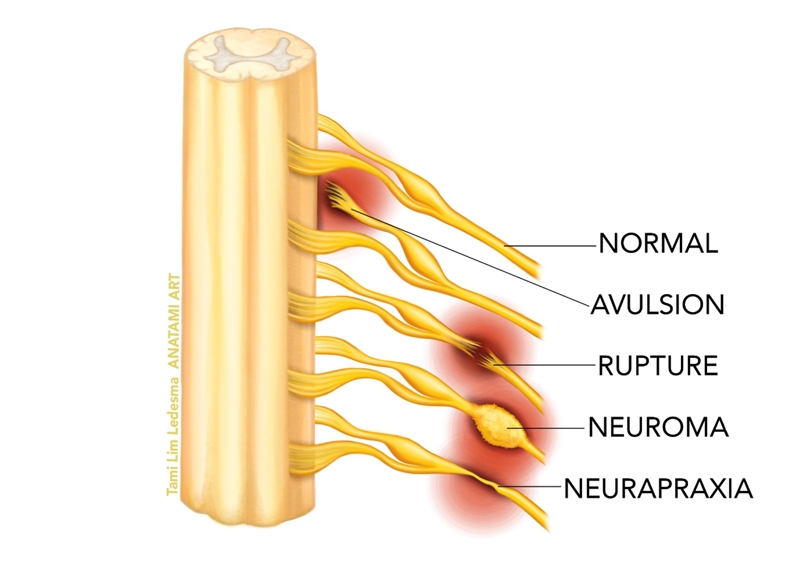
Recapping from part one, brachial plexus injuries (commonly known as‘stingers’ and ‘burners’) typically occur as a result of a fall onto the shoulder (such as falling from a bike or horse), contact on the shoulder (football tackle) or a sudden traction on the raised arm (gymnast on a high bar). These present as a transient and reversible peripheral neuropraxia of all or some of the nerves (usually the upper trunks of the brachial plexus) that comprise the brachial plexus. In more severe cases of contact trauma, the brachial plexus may be stretched, rupture or avulse (see figure 1).
Figure 1: Types of brachial plexus injury
Imaging
When it comes to diagnostic imaging, there are some key points that clinicians should bear in mind:- Imaging and electro diagnostic studies are preferably delayed after injury (3-4 weeks) because pseudomeningoceles (a collection of cerebrospinal fluid around the damaged nerve) indicate asevere BPI, and need a few weeks to form(1-3). Early electromyography (EMG) cannot distinguish between neuropraxia and other more severe degrees of nerve damage(4).
- The magnetic resonance (MR) imaging protocol includes three-dimensional ‘magnetic resonance myelography’ (3D-MRM) to detect preganglionic injuries and ‘magnetic resonance neurography’ (MRN) to evaluate postganglionic lesions.
- More recent imaging modalities such as ‘diffusion tensor tractography’ (DTI) are experimental and require further testing. However, they have already demonstrated high diagnostic accuracy in the assessment of root avulsions(3).
- Computerised tomography myelography is also used for imaging BPI in acute trauma, and is a reliable imaging technique for discovering avulsion injuries.For C8-T1 nerve roots, standard myelography may be preferred, since shoulder anatomy may obscure the CT view(5,6).
EMG
Coupled with imaging techniques such as MR pyelography and CT pyelography, a neurological specialist will conduct EMG studies to evaluate the electrical activity of the muscles affected by a BPI. EMG can help confirm a diagnosis of BPI, localise the level of the lesion, help estimate the severity of axon loss and eliminate other conditions from the differential diagnosis. This approach is particularly useful in detecting isolated nerve lesions to nerves such as the long thoracic (serratus anterior), dorsoscapular (rhomboid) and suprascapular (supraspinatus and infraspinatus).To monitor spontaneous recovery after a closed traction injury, an EMG is recommended every 4–6 weeks. Early electrodiagnostic studies are often unreliable because of the ongoing ‘Wallerian degeneration’, which is typically complete at three to four weeks(7). Repeated physical examinations also help document the progression of recovery, and assess the potential surgical exploration of the brachial plexus(8).
Injury management
Most contact BPIs usually involve a low-gradeneuropraxia, which will spontaneously recover (without major complications) in the first 24 hours after injury. However, sustaining a BPI may make the athlete more prone to future BPI in the form of ‘stingers’ and ‘burners’(9,10). Following a traumatic BPI without spontaneous recovery, it is essential to swiftly diagnose and protect the injured site to prevent further damage and retraction of the nerve.As mentioned, imaging studies and EMG are usually delayed in order to gather a complete and accurate picture of the extent of damage to the brachial plexus. These tests help determine whether or not recovery can be expected and if surgery will be required. In the athletic arena, BPIs requiring surgery are rare. If they do occur, complete nerve ruptures and nerve root avulsions would not recover without surgery. Where surgery is required, severe lesions are managed with nerve grafting, nerve reconstruction, nerve transfers, and free muscle and nerve transplants(11).
Injury grade classification
Treatment of BPI depends heavily upon the classification (grade) of the injury. These are as follows:Grade 1: Neuropraxia
- A less severe injury with conduction disruption around the zone of injury – but with intact axon and preserved supportive structures. Nerve remains in continuity.
- Full recovery usually within a few days to six weeks without surgical intervention.
- After a simple crush injury, function may return within days.
Grade 2: Axonotmesis
- Disrupted axon with intact endoneurium. Wallerian degeneration takes place after 1-2 weeks.
- Prognosis: Variable recovery; worse prognosis for proximal injuries and injuries that do not successfully re-implant in the muscle within 18 months.
Grade 3: Neurotmesis with preservation of perineurium
- Endoneurium is disrupted.
- Prognosis: 60-80% recovery.
Grade 4: Neurotmesis with preservation of epineurium
- Prognosis: Nerve grafting is required. Nerve grafting is a procedure which is used to make connectivity of the ruptured nerves in postganglionic injury(12). Nerve grafting cannot be used in pre-ganglionic injuries, because a length of proximal nerve is required.
Grade 5: Neurotmesis with complete transection of nerve trunk
- Prognosis: Bypass/jump grafting is required.
Factors affecting recovery
Following BPI injury, a number of factors will determine the speed and extent of recovery(13):- Young athletes have a greater potential for recovery than older athletes.
- High-energy injuries have a worse prognosis. These are often difficult to elucidate in the athlete. Usingvideo footage to review the mechanism of injury can be helpful.
- Avulsion injuries have a worse prognosis than ruptures.
- Mixed nerve injuries have less chance of recovery than exclusively motor or sensory nerve injuries.
- Upper trunk lesions have the best prognosis.
- Pre-ganglionic lesions have no potential for spontaneous recovery whereas postganglionic lesions will have potential for spontaneous recovery(7).
Conservative management
For the athlete who suffers a serious BPI and expects a full recovery (based on the results of MR/CT myelography and EMG studies), the anticipated recovery time may be lengthy (up to 1 to 2 years)(14). The recovery of nervous tissue can occur either by axonal regeneration from proximal to distal, or by rein nervation through terminal collateral sprouting. For athletes awaiting spontaneous recovery, intervention helps manage pain and maintain function in the upper limb. Here are a few points regarding injury management in this time-period:- Immobilize the athlete in a sling to prevent shoulder subluxation (in the event of an axillary nerve palsy), and to support the arm.
- Perform daily passive range-of-motion exercises for the scapula, shoulder, elbow, wrist and hand to avoid joint stiffness.
- Manage neuropathic pain with Gabopentin.
- Usedelectrical muscle stimulation on the affected muscles in order to stave off muscle atrophy(15). Due to the deep location of the rotator cuff, and some of the periscapular muscles, gaining access for electrode placement is often very difficult in and around the shoulder.
- Passive stretching (which produces a mild growth stimulus) can also used to prevent gross muscle atrophy(16).
- When EMG shows that some muscle activity has been regained, use gentle and loaded exercises to the target muscles to regain strength.
- Use proprioceptive neuromuscular facilitation(PNF) techniques for global strengthening of the upper limb complex(17-19).
PNF Exercises
Popularized by Dorothy Voss and Maggie Knott in the 1950’s for the rehabilitation of neurological disorders such as stroke and head injury(17),PNF principles may be used in the rehabilitation of extensive nerve lesions such as a BPI. The reader is directed to references 17-19 for a detailed description on how to use PNF techniques in muscle strengthening, but the basics are as follows:Rhythmic initiation
The rhythmic initiation technique involves a progression of initial passive, then active-assistive, followed by active movement against resistance. Through the agonist pattern, movement is slow, goes through the available range of motion, and avoids activation of a quick stretch. Two movement directions are used:
- D1 starts in shoulder extension, internal rotation/abduction with elbow extended and forearm pronated, and moves into shoulder flexion/external rotation/adduction with elbow flexion and supination.
- D2 starts in shoulder flexion/abduction/external rotation and moves into extension/adduction/internal rotation.
This is the easiest and less aggressive of the PNF techniques are used for patients who areunable to initiate movement and who have a limited range of motion because of increased tone. It may also be used to teach the patient a movement pattern.
Repeated contraction
Repeated contraction is useful when a patient has weakness either at a specific point or throughout the entire range. It is used to correct imbalances that occur within the range by repeating the weakest portion of the total range. Using the same D1 or D2 as mentioned above, the patient moves isotonically against maximal resistance repeatedly until fatigue is evidenced in the weaker components of the motion. A stretch at that point in the range should facilitate the weaker muscles and result in a smoother, more coordinated motion.
Slow reversal
Slow reversal involves an isotonic contraction of the agonist followed immediately by an isotonic contraction of the antagonist. The initial contraction of the agonist muscle group facilitates the succeeding contraction of the antagonist muscles. The slow-reversal technique can be used fordeveloping active range of motion of the agonists and normal reciprocal timing between the antagonists and agonists.
References
- Gasparotti R (2012) ‘Radiographic assessment of adult brachial plexus injuries’. In: practical management of pediatric and adult brachial plexus palsies. Kevin C Chung, John E McGillicuddy Lynda, Yang J-S (eds.) Elsevier LTD, London, USA, 234-249
- AJNR Am J Neuroradiol. 1997. 18: 1733-1742
- Invest Radiol. 2013. 48: 104-112
- J Neurol Neurophysiol. 2013. 5:1 DOI: 10.4172/2155-9562.1000180
- J Bone Joint Surgery. 1986. 68B; 734–738
- 1992. 34: 235–240
- J Am Acad Orthop Surg. 2005;13(6):382-396
- 1981. 12(5): 376–382
- The Am J Sports Med. 2015. Vol. 43, No. 11. 2809-2815
- Clin J Sports Med. 2012. 22; 472-477
- J Hand Surgery. 1998. 23A(4): 711–716
- The Nerve.2017.3(1):1-11
- ISRN Orthopedics Volume 2014, Article ID 726103, 9 pagesdx.doi.org/10.1155/2014/726103
- Muscle Nerve. 1990;13(9):771-784
- Muscle Nerve. 2010. 41; 685-693
- J Anatomy. 1999. 194(3); 323-334
- Voss DE, Jonta M, Meyers B (1985) Proprioceptive Neuromuscular Facilitation: Patterns and Techniques, 3rd ed. Harper and Row, New York
- Athl Train. JNATA. 1986;21:26-31
- J Athletic Training. 1997. 32(1). 34-39
You need to be logged in to continue reading.
Please register for limited access or take a 30-day risk-free trial of Sports Injury Bulletin to experience the full benefits of a subscription. TAKE A RISK-FREE TRIAL
TAKE A RISK-FREE TRIAL
Newsletter Sign Up
Subscriber Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Newsletter Sign Up
Coaches Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Be at the leading edge of sports injury management
Our international team of qualified experts (see above) spend hours poring over scores of technical journals and medical papers that even the most interested professionals don't have time to read.
For 17 years, we've helped hard-working physiotherapists and sports professionals like you, overwhelmed by the vast amount of new research, bring science to their treatment. Sports Injury Bulletin is the ideal resource for practitioners too busy to cull through all the monthly journals to find meaningful and applicable studies.
*includes 3 coaching manuals
Get Inspired
All the latest techniques and approaches
Sports Injury Bulletin brings together a worldwide panel of experts – including physiotherapists, doctors, researchers and sports scientists. Together we deliver everything you need to help your clients avoid – or recover as quickly as possible from – injuries.
We strip away the scientific jargon and deliver you easy-to-follow training exercises, nutrition tips, psychological strategies and recovery programmes and exercises in plain English.









