Neuromuscular electrical stimulation: An underutilized modality that may aid in bridging the gender bias in ACLR recovery

Anterior cruciate ligament (ACL) injuries occur two to eight times more often in females than males(1,2). While ACL injuries often happen while playing contact sports, over 70% of them result from noncontact situations(1). This fact is especially true for females for whom most injuries occur during maneuvers such as cutting, pivoting, or landing from a jump(1,3).
Not only are females at greater risk for initial ACL injury, but the likelihood of future injury further increases following ACL reconstruction (ACLR). Females who undergo ACLR are 16 times more likely than healthy female control subjects to suffer an additional ACL injury(4). When compared to male athletes, the female rate of injury following ACLR is six times greater(4).
Females may have a different response than males to standard ACLR physical therapy protocols(5). They are significantly weaker on a quadriceps index than males six months postoperatively, resulting in less readiness for return to sport(5). The difference in strength recovery may indicate a need for greater emphasis on gender-specific rehabilitation(6).
The gender bias that exists for ACL injury risk and recovery is likely multifactorial. Intrinsic factors include hormonal and structural differences, while extrinsic factors include neuromuscular and biomechanical variables (see table 1)(1,2,3). Since extrinsic factors like quadriceps strength are modifiable, physios should ensure optimal utilization of available interventions to help facilitate improved outcomes in these areas.
| INTRINSIC FACTORS | EXTRINSIC FACTORS |
|---|---|
| Joint and Ligamentous Laxity | Muscular Strength |
| · The female ACL is less stiff (lower modulus of elasticity), contributing to joint laxity, and fails at a lower load level (lower failure strength).· Females’ elastin and collagen tissue may contribute to the completeness of ACL tears and scar formation. | · After normalization for body weight, females are weaker than males in hip, quadriceps, and hamstring strength, contributing to reduced lower extremity joint stabilization.· Males can generate higher forces in leg extensor muscles and produce those forces more quickly than females. |
| Hormonal Influences | Muscular Activation Patterns |
| · Women have more body fat and less lean body mass than males because of increased estrogens in the female and increased androgens in the males.· There are estrogen receptors within the ACL. The ligament’s mechanical and molecular properties are likely influenced by estrogen and the interaction of several sex hormones, secondary messengers, remodeling proteins, and stresses.· Fluctuations in hormones that occur throughout the menstruation cycle may increase ligament laxity and decreased neuromuscular control. | · Females exhibit different quadriceps, hamstrings, and gastrocnemius muscle activation patterns than males. |
| Structural Difference | Jumping and Landing Mechanics |
| · Females have a wider pelvis, are more flexible, and have less developed musculature than men.· Lower extremity alignment differs in the female and may predispose them to injury. | · Females tend to be in a more upright posture with less hip and knee flexion and their knees in more valgus angulation during cutting, landing, and squatting. |
NMES and arthrogenic muscle inhibition
One such intervention is the use of neuromuscular electrical stimulation (NMES) for improving quadriceps activation. Quadriceps weakness following ACLR is a routine finding regardless of gender and can be a significant barrier to effective rehabilitation. This muscle weakness is often due to a phenomenon called arthrogenic muscle inhibition (AMI), whereby muscle contraction is limited despite no structural damage to the muscle or innervating nerve(7). Rather, mechanisms of muscular inhibition include alteration in muscle resting motor thresholds, changes in the discharge of articular sensory receptors, altered spinal reflex excitability, and abnormal cortical activity(8). Failing to address AMI is associated with long-term quadriceps atrophy, gait abnormality, poor function, dynamic instability, persistent knee pain, and early osteoarthritis(8).While most rehabilitation programs address AMI through therapeutic exercise, neuromuscular electrical stimulation (NMES), in addition to therapeutic exercise, may be more effective in improving quadriceps strength than exercise alone(9,10). When performed immediately postoperatively, NMES reduces skeletal muscle fiber atrophy and conserves contractility, often markedly reduced after surgery(11). Its use may also facilitate more rapid achievement of a higher quadriceps index, which allows for the earlier progression of training activities, i.e., agility drills(10). A quicker return to sport-specific training is especially important for female athletes who require more extensive training of other extrinsic factors, such as jumping and landing mechanics, before making a safe return to play.
Parameters
Unfortunately, NMES remains controversial and underutilized largely due to the lack of a standardized protocol. A systematic review of 8 randomized control trials found no consistent parameter set up, varying treatment duration from three to 11 weeks, and a range of treatment sessions from 12 to105(9). With so much variability, it is understandable why many physios shy away from the unknown. While a standardized protocol is pending, a summary of the literature does provide parameters based on current evidence (see table 2).| PARAMETER | RECOMMENDATION BASED ON SYNTHESIS OF LITERATURE |
|---|---|
| Limb Position | ~65 degrees knee flexion; less knee flexion may be used if better tolerated but is not as effective |
| Electrode Placement | No standardized location is reported in the literature; areas used include: |
| · Proximally: femoral nerve or muscle belly of rectus femoris or vastus intermedius or vastus lateralis | |
| · Distally: motor point of muscle belly of vastus medialis | |
| NMES waveform | Low-frequency biphasic or medium-frequency bust-modulated AC |
| Frequency | 30-50 Hz PC or 2500 Hz AC in 50 Hz bursts |
| Pulse Duration: | 250-400 µs |
| Current Amplitude | Individual maximally tolerated intensity; minimum is strong but comfortable muscle contraction |
| Work:Rest/On:Off Cycle | 6-10:12-50 seconds; use lower duty cycle (i.e. 1:3 or 1:5) if the muscle is weaker to limit fatigue |
| Treatment Schedule | Initiate ideally within 1 week postoperatively |
| Session Frequency | 3x/week over 4-6 weeks, particularly in the first 6 weeks postoperatively; may start as soon as postoperative day 1 |
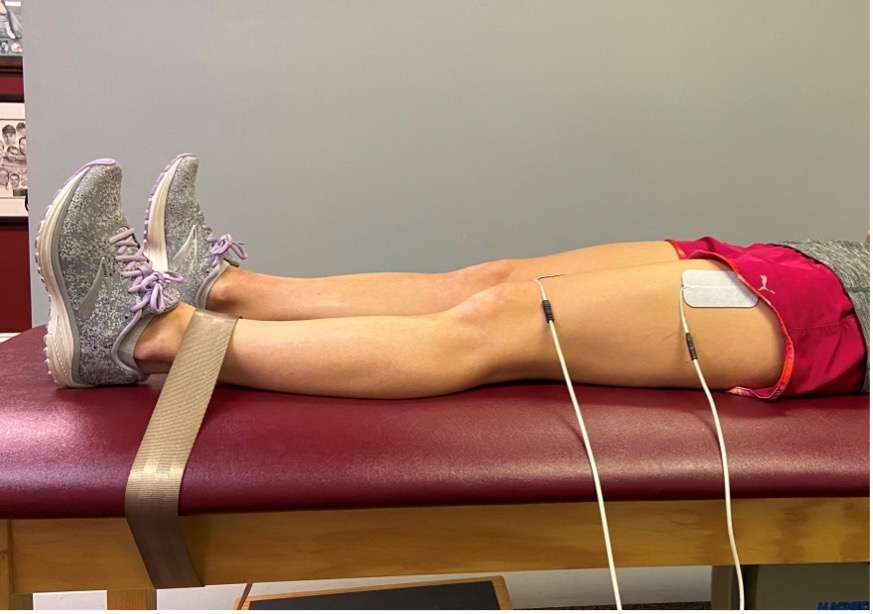
Position the athlete with their knee in approximately 65 degrees of flexion (the resting length of the quadriceps) to facilitate maximal force production and optimal outcomes(12). Ideally, use an isokinetic machine or dynamometer to prevent any net extension torque(13). Otherwise, a patient can sit in a knee extension machine with the weight stack fully loaded to facilitate an isometric contraction. (see figure 1). If an athlete does not tolerate this amount of flexion in the early post-op phase, position them supine with 30 degrees of knee flexion using a bolster and belt (see figure 2). However, keep in mind that knee flexion less than 30 degrees produces inferior outcomes(12). If the patient experiences patellar discomfort with the knee in flexion (a common occurrence with patellar tendon autografts), position the knee in full extension (see figure 3)(10); however, use caution as this position places a high strain on the ACL(12).
Figure 1. Position of use at 65 degrees of knee flexion
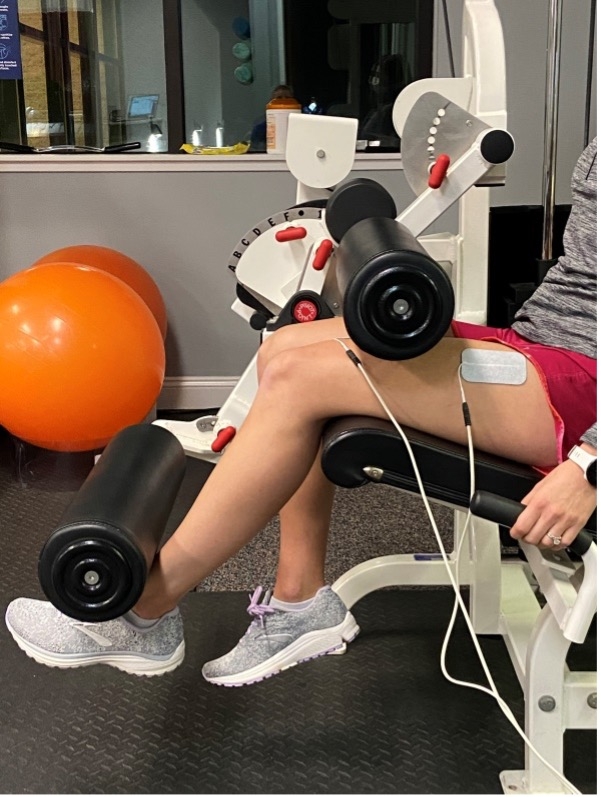
Figure 2. Position of use at 30 degrees knee flexion
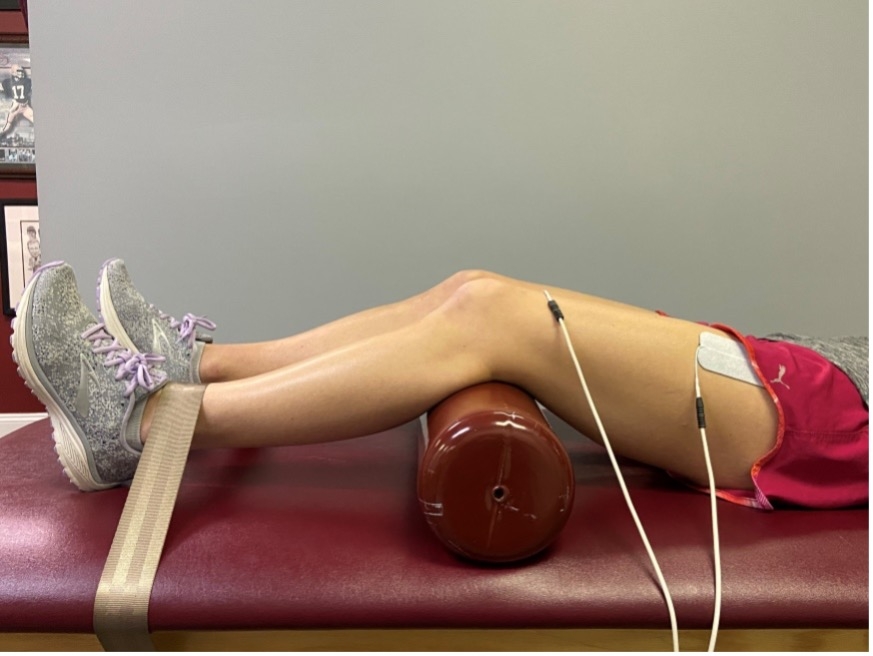
Figure 3. Position of use at 0 degrees knee flexion
Place the electrodes over motor points to minimize the electrical current needed to elicit a muscle contraction(14).Electrode placement on other sites requires a higher intensity current to reach the motor branch and may cause discomfort from the excitation of sensory fibers(14). Accordingly, larger electrodes generally result in a greater likelihood of appropriate placement and greater comfort for the patient.
Intensity should be set to maximal tolerance but must be high enough to elicit a muscular contraction(12). Adjust the duty cycle based on the athlete’s muscular endurance, which may vary based on the time since surgery and individual differences. To limit fatigue in those with considerable muscular strength and endurance deficits, use a lower duty cycle of 1:3 or 1:5. Time treatments to elicit a minimum of 10 muscular contractions per session(10,15).
Key takeaways
- Females are at greater risk than males for ACL injury and reinjury based on intrinsic and extrinsic factors(1,2,3).
- Females exhibit more significant quadriceps strength deficits than males following ACLR, which results in decreased readiness and likelihood of a successful return to play(5).
- Following ACLR, NMES combined with therapeutic exercise is more effective at improving quadriceps strength than therapeutic exercise alone(9,10).
- The use of NMES is indicated post-ACLR to augment volitional recruitment and strength of the quads and might be especially beneficial in the treatment of female athletes(12).
References
- J Orthop Sports Phys Ther 2007;37(2):A1–A32.
- All Volumes. 2006; 64.
- Clin Sports Med. 2004; 23:281-298.
- Clin J Sport Med. 2012;22(2):116-121
- 55th Annual Meeting of the Orthopedic Research Society: Poster No. 1954.
- Biomed Sci Instrum. 2005;41(323-328).
- J Neuroeng Rehab. 2012 Jul 12;16(1):89.
- Br J Sports Med. 2019;53:289-298.
- J Orthop Sports Phys Ther. 2010;40(7):383-391.
- J Orthop Sports Phys Ther. 2003;33(9):492-501.
- J. Sports Med. 2020;48(10): 2429–2437.
- Physiother Can. 2017;69(5):1-76.
- Phys Ther. 1988 May;68(5):660-3.
- J Neuroeng Rehab. 2014;11(17).
- Biomed Res Int. 2013;2013:802543.
You need to be logged in to continue reading.
Please register for limited access or take a 30-day risk-free trial of Sports Injury Bulletin to experience the full benefits of a subscription. TAKE A RISK-FREE TRIAL
TAKE A RISK-FREE TRIAL
Newsletter Sign Up
Subscriber Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Newsletter Sign Up
Coaches Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Be at the leading edge of sports injury management
Our international team of qualified experts (see above) spend hours poring over scores of technical journals and medical papers that even the most interested professionals don't have time to read.
For 17 years, we've helped hard-working physiotherapists and sports professionals like you, overwhelmed by the vast amount of new research, bring science to their treatment. Sports Injury Bulletin is the ideal resource for practitioners too busy to cull through all the monthly journals to find meaningful and applicable studies.
*includes 3 coaching manuals
Get Inspired
All the latest techniques and approaches
Sports Injury Bulletin brings together a worldwide panel of experts – including physiotherapists, doctors, researchers and sports scientists. Together we deliver everything you need to help your clients avoid – or recover as quickly as possible from – injuries.
We strip away the scientific jargon and deliver you easy-to-follow training exercises, nutrition tips, psychological strategies and recovery programmes and exercises in plain English.








