Divide and conquer: using subgroups to manage Achilles tendinopathy

Achilles tendinopathy (AT) is one of the most common overuse injuries, with a prevalence rate of approximately 10%(1). The injury usually occurs following high-level repetitive loading, such as prolonged running or jumping activities. As a result, athletes often report a reduction in participation, impaired performance, and quality of life (QoL)(2).
Due to the insidious nature of AT, athletes may delay seeking therapeutic management. The pain usually presents at rest and as early morning stiffness (EMS). Initially, athletes may train with some degree of discomfort as the pain subsides with activity. The Achilles tendon is a traction tendon, meaning that the direction of pull is in line with the muscle(3). Therefore, load from the calf musculature is transmitted directly into the tendon fibers.
| 1. An increase in physical activity2. Number of years of running3. Reduced recovery time between training sessions4. Change of footwear5. Change of training surface/ground6. Calf muscle weakness or reduced endurance7. Poor lower leg muscle flexibility8. Excessive foot pronation |
| 2. Number of years of running |
| 3. Reduced recovery time between training sessions |
| 4. Change of footwear |
| 5. Change of training surface/ground |
| 6. Calf muscle weakness or reduced endurance |
| 7. Poor lower leg muscle flexibility |
| 8. Excessive foot pronation |
Tendon Injury Classification
Achilles tendon injury can occur at three different locations: the musculotendinous junction, the midportion, or the insertion site (see figure 1).Figure 1: Achilles tendon injury sites.
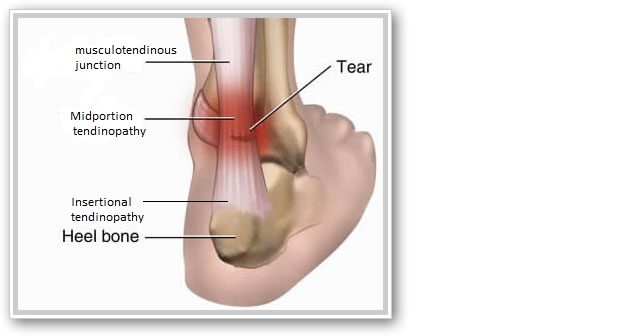
A multi-stage continuum model exists to classify tendon injuries(4). This model identifies three stages: reactive tendinopathy, tendon disrepair, and degenerative tendinopathy. The first stage, reactive tendinopathy, occurs due to an acute increase in load to the tendon, e.g., increased physical activity, reduced rest days, or altered training type such as switching from steady runs to hill climbs. As a result, the tendon thickens because of proliferative, non-inflammatory cell influx to the area. The influx of cells is an attempt to increase the cross-sectional area (CSA) and, therefore, better handle the load. The tendon is therefore structurally intact, and the damage is reversible(4).
Tendon disrepair occurs when the tendon is subject to continual overload. The tendon thickens further to heal itself, accompanied by other cell migration to the region, causing a breakdown and disorganization of the collagen matrix. As a result, there is an alteration in tendon structure, but healing is still possible with appropriate treatment(4).
The final stage is degenerative tendinopathy. Chronic overload causes multiple structural changes such as areas of cell death, increased vascularization, and extensive collagen dissemination. Pathological cellular disorganization interspersed with earlier stages of damage and normal tendon is characteristic of the final stage, and these changes are irreversible(4).
Subgroup classification
The Achilles tendon has to absorb loads of up to seven times body weight upon impact(5). Therefore current treatment protocols suggest progressive loading through eccentric exercises or heavy slow resistance training(6,7). These loading protocols can appear prescriptive and offer a one-size-fits-all approach, which is perhaps why up to 40% of patients experience a reoccurrence following rehabilitation. Both methods involve taking the load to a level that meets the tendon’s demands and requires three to six months of progressive loading(8). The variability in rehabilitation time may be due to the athlete’s apprehension due to pain or fear of causing further damage. For these reasons, re-injury rates can be high. Athletes may return to play prematurely before restoring adequate tissue capacity and are underprepared for the demands of their sport.Researchers at the University of Delaware provide a change in the standardized treatment approach by identifying subgroups in AT. Investigators evaluated five key variables that represent complete tendon health(9). The assessment provides a comprehensive biopsychosocial evaluation of the patients with AT (see table 1).
| Variable | Test |
|---|---|
| Symptoms | · The Victorian Institute of Sport Assessment (VISA-A) |
| · Single leg hopping test | |
| · Numerical pain rating scale of 0-10 | |
| Lower extremity function | · Single-leg countermovement jump |
| · Drop countermovement jump | |
| · Heel-rise endurance test | |
| · Physical Activity Scale (PAS) | |
| Patient-related factors | · Body mass index, age, sex |
| · Foot and Ankle Outcome Score (FAOS) | |
| · Duration of symptoms | |
| · Injury side and location | |
| Psychological factors | · Tampa Scale of Kinesiophobia (TSK-17) |
| · Pain Catastrophizing Scale (PCS) | |
| Tendon structure and mechanical properties | · Ultrasound imaging (cross-sectional area, thickness, and viscosity) |
| · Shear wave elastography (mechanical properties) |
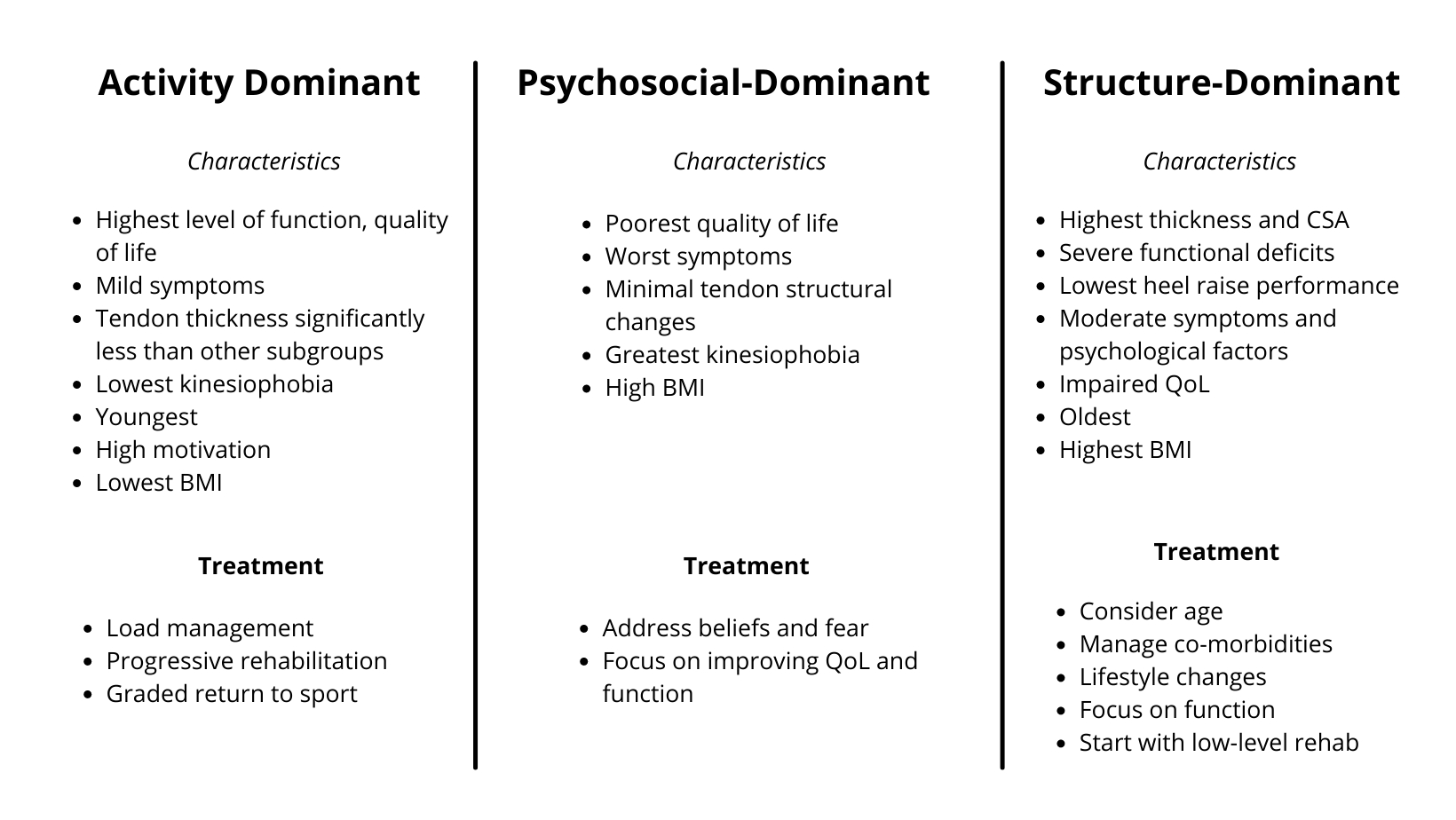
Investigators used the five variables to formulate the different patient subgroups and consider all domains of tendon and patient health. The three subgroups explain the variability in treatments, timescale to recovery, obstacles to recovery, and patient outcomes (see figure 2).
Figure 2: Subgroups in Achilles Tendinopathy(9).
Clinical implications
The identification and classification of clients into subgroups provides clinicians with pragmatic treatment strategies. Each group will require a different management approach, and this may improve outcomes. Once identified, athletes can be managed and educated accordingly.- Activity-dominant
This group is likely to respond well to standard tendinopathy treatment, including education, load management, and progressive rehabilitation. The prognosis is favorable with 12 weeks of rehabilitation(9). The activity-dominant subgroup is typically physically active and highly motivated.
- Psychosocial-dominant
This subgroup requires a holistic approach that focuses on their fear and beliefs associated with their tendon health. Kinesiophobia may prove to be a barrier to rehabilitation success as tendon loading is essential for restoring function. To allow for the progression of rehabilitation and function, clinicians should aim to identify athlete fears and provide education to enhance their understanding. Exercises should focus on restoring function to demonstrate that progress can occur and equally improve athlete’s QoL and lessen the psychological burden(9).
- Structure-dominant
Clinicians should identify the functional deficits and commence loading at a low level. The intensity of the initial rehabilitation program should be sub-threshold. Once the tissue load tolerance is determined, rehabilitation can slowly progress. Clinicians must consider an athlete’s overall health and thus, appropriately manage co-morbidities. Weight management, nutrition, and referral to other medical professionals to manage existing co-morbidities may be required. This subgroup presents with the highest BMI, which may explain the poor tendon health and performance as obesity and co-morbidities would impact tendon loading and healing, respectively. Individuals may be generally de-conditioned, and a holistic rehabilitation plan would increase their QoL and allow progress over time(9).
Conclusion
Classifying aspects of tendon health into five variables can simplify the assessment process for AT sufferers. According to five variables, clinicians may classify athletes into one of three subgroups. Classification into subgroups can assist athlete rehabilitation plans, dealing with expectations, identifying predisposing factors, and enhancing outcomes. The systematic classification of athletes into subgroups provides clinicians with an evidence-based framework to build a comprehensive management plan. Future research may offer similar frameworks to other common sports injuries.References
- Sports Med 2012; 42 (10): 891-905
- Br J Sports Med 2021;55:486–492.
- Scand J Med Sci Sports. 2005 Aug: 15(4); 211-222.
- Br J Sports Med. 2009; 43(6): 409-416.
- Phys Ther in Sport. 2012 June; 13:3-10.
- Sports Medicine. 2013; 43(4), 267-286.
- American J Sports Med. 2015; 43(7): 1704-1711.
- Br J Sports Med. 2018; 52(10): 622-623.
- J Orthop Sports Phys Ther. 2021; 1(1):1-28.
You need to be logged in to continue reading.
Please register for limited access or take a 30-day risk-free trial of Sports Injury Bulletin to experience the full benefits of a subscription. TAKE A RISK-FREE TRIAL
TAKE A RISK-FREE TRIAL
Newsletter Sign Up
Subscriber Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Newsletter Sign Up
Coaches Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Be at the leading edge of sports injury management
Our international team of qualified experts (see above) spend hours poring over scores of technical journals and medical papers that even the most interested professionals don't have time to read.
For 17 years, we've helped hard-working physiotherapists and sports professionals like you, overwhelmed by the vast amount of new research, bring science to their treatment. Sports Injury Bulletin is the ideal resource for practitioners too busy to cull through all the monthly journals to find meaningful and applicable studies.
*includes 3 coaching manuals
Get Inspired
All the latest techniques and approaches
Sports Injury Bulletin brings together a worldwide panel of experts – including physiotherapists, doctors, researchers and sports scientists. Together we deliver everything you need to help your clients avoid – or recover as quickly as possible from – injuries.
We strip away the scientific jargon and deliver you easy-to-follow training exercises, nutrition tips, psychological strategies and recovery programmes and exercises in plain English.










