Uncommon injuries: Don't play with groin pain - femoral neck stress fractures

First reported by Asalin, a German military surgeon in 1905, a femoral neck stress fracture (FNSF) is a relatively uncommon injury(1). Estimates vary, but in healthy young subjects suffering a stress fracture, FNSFs account for between 1% and 7.2% of such lesions(2). Like all stress fractures, most FNSFs in athletes are fatigue-induced fractures, which are caused by repetitive loading of healthy bone with unsustainable (for the athlete) forces(3). However, unlike many other stress fractures, FNSFs are often not amenable to conservative management techniques and require surgery.
What is a femoral neck stress fracture?
The neck of the femur acts as a lever and transmits forces generated by the muscles of the thigh and pelvis to the trunk via the hip joint. Due to its profile and orientation, it is subject to considerable compressive and tensile forces during activities such as running (see figure 1). In most cases, stress from tensile forces leads to a tension fracture, which originates on the upper aspect of the neck. By contrast, a fracture resulting from compressive forces tends to occur on the lower side of the neck.Figure 1: FNSF location and compressive/tensile forces
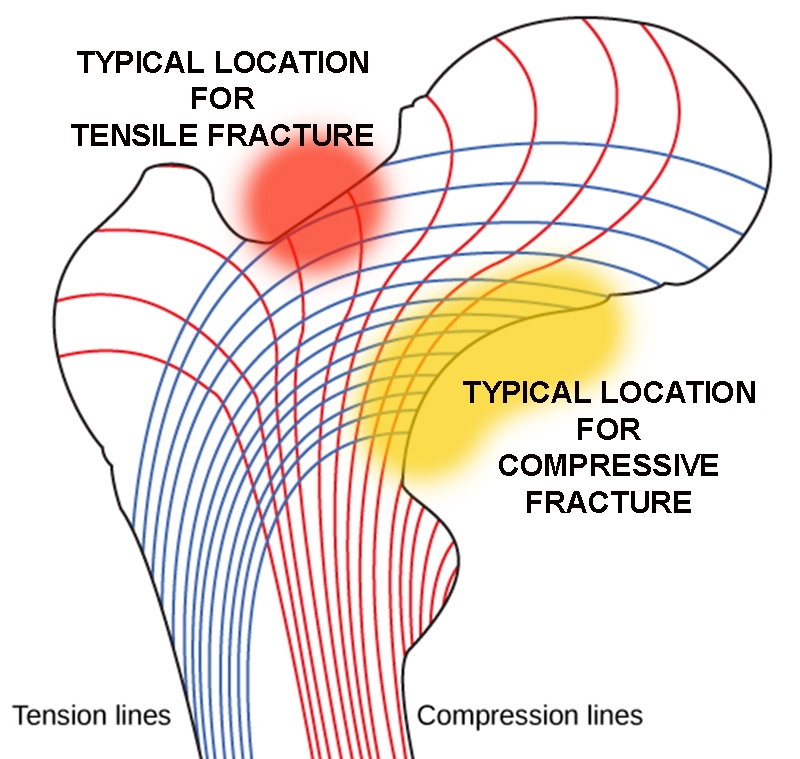
Tension = blue lines; compression = red lines. Fracture risk increases where force lines run parallel to the neck surface.
Femoral neck stress fractures are classified according to the origin, severity and progression of the fracture. One of the earliest classification systems is the Garden classification of subcapital femoral neck fractures, which classifies femoral neck fractures into four types based on the displacement of the femoral head (see figure 2)(4). Developed when CT and MRI imaging was unavailable, this system has limitations as many femoral neck fractures classified as Type I on plain radiographs may be Type II or III on CT or MRI imaging. Despite these limitations, however, the classification is still well accepted and widely used by orthopedic surgeons(5).
Figure 2: The Garden system of FNSF classification
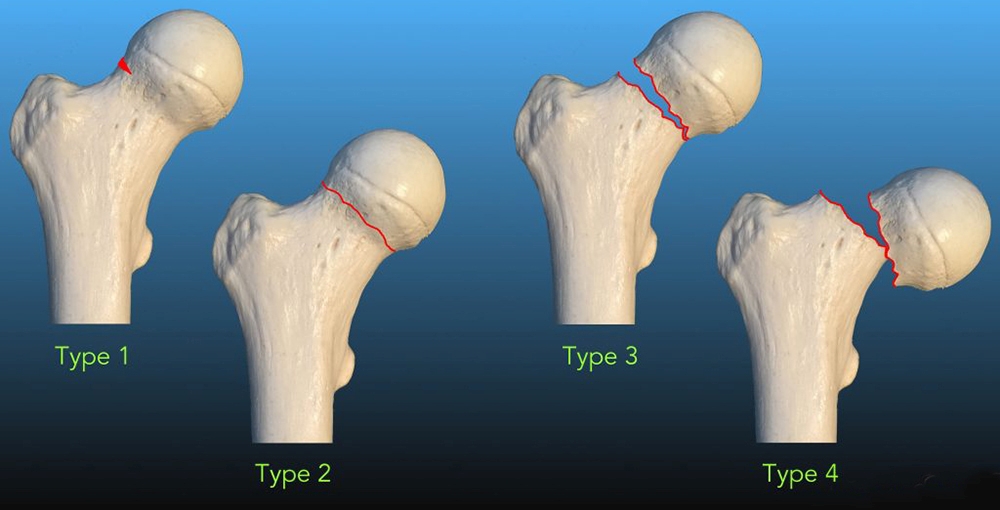
*Garden type I: nondisplaced incomplete
*Garden type II: nondisplaced complete
*Garden type III: complete fracture, incompletely displaced
*Garden type IV: complete fracture, completely displaced
In general, type I and II are stable fractures and are treated with internal fixation (head-preservation). In contrast, type III and IV are unstable fractures and hence treated with arthroplasty.
Currently, the most commonly used classification is the Fullerton and Snowdy classification(6). This system classifies fractures into one of three categories (based on X-ray and bone scans):
- Type I – originating on the tension-side of the femoral neck
- Type II – originating on the compression side of the neck
- Type III – all displaced fractures
Fullerton and Snowdy determined that fractures resulting from tension on the lateral aspect of the femoral neck present a higher risk than compression fractures as they are inherently unstable and prone to displacement. By contrast, medial femoral neck fractures resulting from compressive forces present a lower risk, and can (depending on the extent of the breach) be amenable to conservative treatment methods.
The etiology of FNSF
Although there is a general perception that stress fractures affect male and female athletes equally, more recent research suggests that due to physiological and biochemical differences, women are more vulnerable. Femoral neck stress fractures occur more often in young female runners, endurance athletes, and military recruits (as opposed to their male contemporaries)(7).Repeated loading of the femoral neck that exceeds the body’s ability to adequately remodel the affected bone tissue and replace the old/damaged bone with new bone results in an area of weakened bone. This area of bone is then vulnerable to fracture. It follows, therefore, that any nutritional factors that might impede efficient bone remodeling will, over time, lead to an increased risk for an FNSF (8). Some of the dietary/lifestyle risk factors associated with an increased FNSF risk in athletes, especially females, are:
- Limited calorie intake, dairy-free, and low-fat diets, and eating disorders during adolescence(9,10).
- Amenorrhea (2-4 times higher risk of FNSFs compared eumenorrheic athletes)(11).
- Low vitamin D status(12).
- Reduced femoral bone mineral density (FNSFs are 2.6 times more common for every reduction of one standard deviation of femoral bone mineral density)(13).
Many of these risk factors may be rooted in sub-optimum nutrition. Eagle-eyed clinicians will recognize how many of these are typical characteristics of relative-energy deficiency syndrome (RED-S – see this article for a more in-depth discussion). A careful history, therefore, should include the athlete’s current and past dietary intake and habits. Clinicians should also inquire about the athlete’s training history, noting any significant increase in volume or intensity in recent months. A menstrual history is also imperative for female athletes with suspected FNSF.
Diagnosis
The presentation of an FNSF in an athlete is often vague and non-specific(14,15); indeed, evidence suggests that around 75% of FNSFs are either misdiagnosed or undiagnosed following a physical examination(16). Athletes may complain of groin pain yet continue playing through the pain. The typical presenting symptoms in an athlete with FNSFs are as follows:- Gradual onset of groin or hip pain, which increases with activity and weight-bearing, possibly causing a limp during walking.
- Pain that lessens or resolves with non-weight bearing rest.
- Pain in the anterior groin area, thigh, gluteal region, or which radiates to the knee.
- Pain that increases with higher exercise duration or intensity.
- Pain that manifests towards the end of exercise may signify the early stages of an FNSF.
- Pain that persists throughout training, including during rest and sleep, means a worsening or later stage injury.
- In cases where a fracture is complete or displaced, athletes may report a popping or cracking sensation during exercise(17,18).
Immediately refer athletes suspected of suffering an FNSF for imaging. While X-ray imaging is a useful first step, its scope is limited, having a low sensitivity for early detection (15%) and at only 50% sensitivity at follow-up(19). X-ray imaging is not sufficient to accurately diagnose an FNSF. Not only does it frequently fail to show any clear findings when an FNSF is present, it often portrays complete FNSFs as incomplete fractures(20).
Magnetic resonance imaging is the gold standard for obtaining a correct and early diagnosis – essential to preventing an incomplete fracture from becoming complete or displaced. Compared to an X-ray, MRI is better able to diagnose the presence and nature of the fracture(21). Its sensitivity means it can detect early, low-grade bony changes associated with FNSFs, such as periosteal, muscle, and bone marrow edema, thus improving early detection(12).
Treatment options
Although often involving surgery, the treatment options depend on imaging findings. In the case of a nondisplaced, compression side, incomplete fracture, athletes may try four weeks of conservative management completely non-weight bearing on that leg. Subsequent and regular imaging is needed to assess bone healing. With documented bone repair and resolution of pain, the athlete can begin graduated weight-bearing.Physical therapy at this stage should include cross-training activities with the upper extremity to preserve cardiovascular fitness. Suggested activities include using an upper extremity ergometer, medicine ball drills in sitting, and high reps of low-load upper extremity resistance activities. Resistance training on the contralateral leg takes advantage of the neuroplasticity principle of cross-over and builds strength on the injured side. Progress weight-bearing as per the orthopedic doctor’s recommendation.
Continued imaging must confirm continual bone healing with progressive weight-bearing and activity. If athletes still experience pain after four weeks of rest, surgical fixation is recommended. Simultaneously address nutritional deficits including poor diet and low vitamin D status.
Figure 3: Surgically repaired FNSF using a metal screw-plate fixation
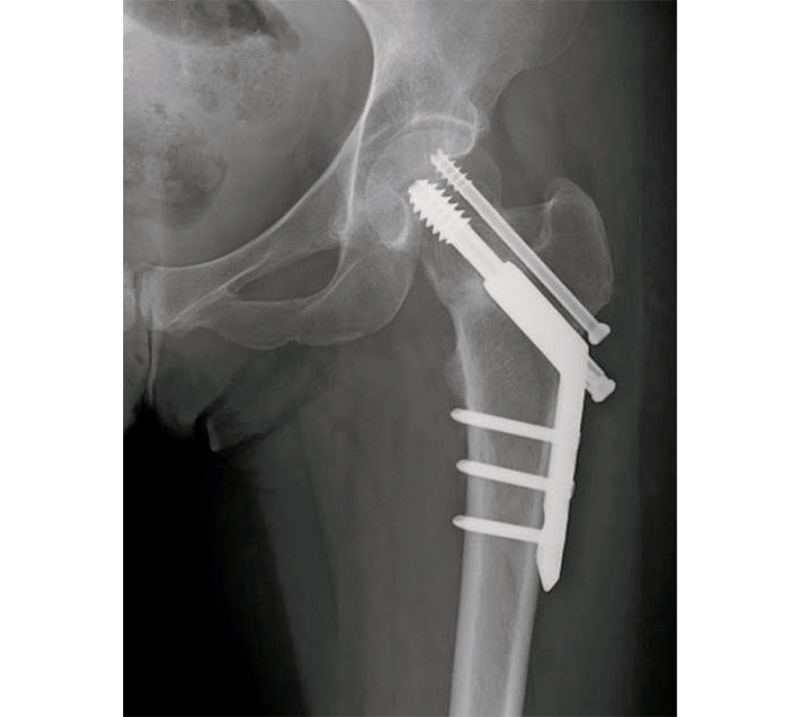
In the case of tension-side fractures, and all complete and displaced fractures, surgery is recommended. Surgeons correct FNSFs with metal-constructed internal fixation devices. Currently, the best surgical option is considered to be the screw-plate fixation (see figure 3). This type of fixation provides superior support for axial loading compared to cannulated screws or intramedullary nailing(22). In the longer term, athletes with poor nutritional habits should be counseled by a qualified dietician as to the importance of nutrition for bone health and minimizing the risk of a future stress fracture.
References
- Belcher A. Ueber den Einfluss des Parademarches auf die Entstehung der Fussgeschwulst. Med Klin 1905; 1: 305-306
- Arch Phys Med Rehabil 1999; 80: 236-38
- Sports Health 2013; 5: 165-174
- J Bone Joint Surg Br. 1961;43:647–663
- Clin Orthop Relat Res. 2018;476(2):441–445
- American Journal of Sports Medicine 16.4(1988): 365-377
- Curr Osteoporos Rep. 2006 Sep; 4(3):103-9
- American J Sports Med 2004; 32(6): 1528-1534
- American J Clin Nutr 1990;51(5): 779-783
- Bennell K., Malcolm SA, Thomas SA, Ebeling PR, McCrory PR, Wark JD, Brukner PD. Risk factors for stress fractures in female track-and-field athletes: a retrospective analysis. 1995
- Sport Med 1999; 28: 91-122
- Acta Biomed 2017; Vol. 88, Supplement 4: 96-106
- Br Med J 1996; 312: 1254-1259
- Acta Orthop Scand 2002; 73(3): 359-68
- Eur J Orthop Surg Traumatol 2012; 22 Suppl. 1: 103-6
- American journal of sports medicine 44.8 (2016): 2122-2129
- Research in Sports Medicine 2016; 24(3): 283-297
- J American Academy of Orthopaedic Surgeons 5.6 (1997): 293-302
- Operative Techniques in Sports Medicine 17.2 (2009): 90-93
- Military medicine 2015; 180(1): e134-e137
- Bone. 2012 Nov;51(5):929-32
- Am J Sports Med 1996; 22: 168-176
You need to be logged in to continue reading.
Please register for limited access or take a 30-day risk-free trial of Sports Injury Bulletin to experience the full benefits of a subscription. TAKE A RISK-FREE TRIAL
TAKE A RISK-FREE TRIAL
Newsletter Sign Up
Subscriber Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Newsletter Sign Up
Coaches Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Be at the leading edge of sports injury management
Our international team of qualified experts (see above) spend hours poring over scores of technical journals and medical papers that even the most interested professionals don't have time to read.
For 17 years, we've helped hard-working physiotherapists and sports professionals like you, overwhelmed by the vast amount of new research, bring science to their treatment. Sports Injury Bulletin is the ideal resource for practitioners too busy to cull through all the monthly journals to find meaningful and applicable studies.
*includes 3 coaching manuals
Get Inspired
All the latest techniques and approaches
Sports Injury Bulletin brings together a worldwide panel of experts – including physiotherapists, doctors, researchers and sports scientists. Together we deliver everything you need to help your clients avoid – or recover as quickly as possible from – injuries.
We strip away the scientific jargon and deliver you easy-to-follow training exercises, nutrition tips, psychological strategies and recovery programmes and exercises in plain English.










