Tendinopathy: inflammation, degeneration and treatment
Alicia Filley looks at the treatment options for tendon injuries based on the inflammation & degeneration paradigm approach.

Tendons are bands of tough connective tissue at the ends of muscles that transfer forces between muscles and bones to move joints and maintain posture. They store kinetic energy, absorb shock, and aid in proprioception. To endure the great forces placed upon them, tendons must be strong and rigid, yet flexible enough to participate in joint movement. Tenocytes, the regulatory cells within the tendon, synthesize all the components in the extracellular matrix, including collagen. Collagen provides the tendon’s tough yet elastic structure through bundles of molecules formed into concentric fibrous bands.
Healing in healthy tendons is divided into three stages(1)
- The initial response after strain is inflammation, which provides clotting to ruptured vessels within the tendon, and consumes the tissue that is no longer viable.
- In the proliferative and repair stage that follows, fibroblasts and tenocytes rush to the wound site, multiply, and rebuild the structure of the tendon.
- The remodeling phase begins one to two months after injury when the collagen fibers align in the direction of stress along the tendon. Through this process, tendons adapt and grow in thickness and length.
Historically, symptomatic tendons that failed to heal were assumed stuck in a cycle of inflammation, and the condition was labeled tendonitis. Subsequent research has found evidence of degeneration within symptomatic tendons, and the inflammatory theory was subsequently discarded in favor of the term ‘tendinosis’. More recent work suggests that both inflammation and degeneration exist in failed tendon healing, called tendinopathy.
| Intrinsic factors | Extrinsic factors |
| Age — advanced age increases risk Gender —males have a greater incidence of tendinopathy and rupture Genetics Bony alignment Functional movement patterns Strength Flexibility | Shoes and other footwear Faulty techniqueRapid changes in training volume or work without sufficient recovery Ill-fitting equipment Training surfaces Fatigue |
Tendinopathy
When excessive loads, along with intrinsic or extrinsic factors, inhibit a normal healing response to strain, the tendon becomes symptomatic (see box 1). Researchers in Melbourne have called this stage ‘reactive tendinopathy’, and describe the proliferation of collagen within the tendon as ‘non-inflammatory and short-term’(2). If the strain on the tendon remains, the tendon further attempts to adapt by increasing the protein molecules in the extracellular matrix and sprouting new blood vessels in the tendon, termed tendon disrepair. Continued strain leads to tendon degeneration. However, if the load is modified before the tendon reaches the degenerative stage, the tendon may have the ability to recover through a normal healing response (see figure 1).
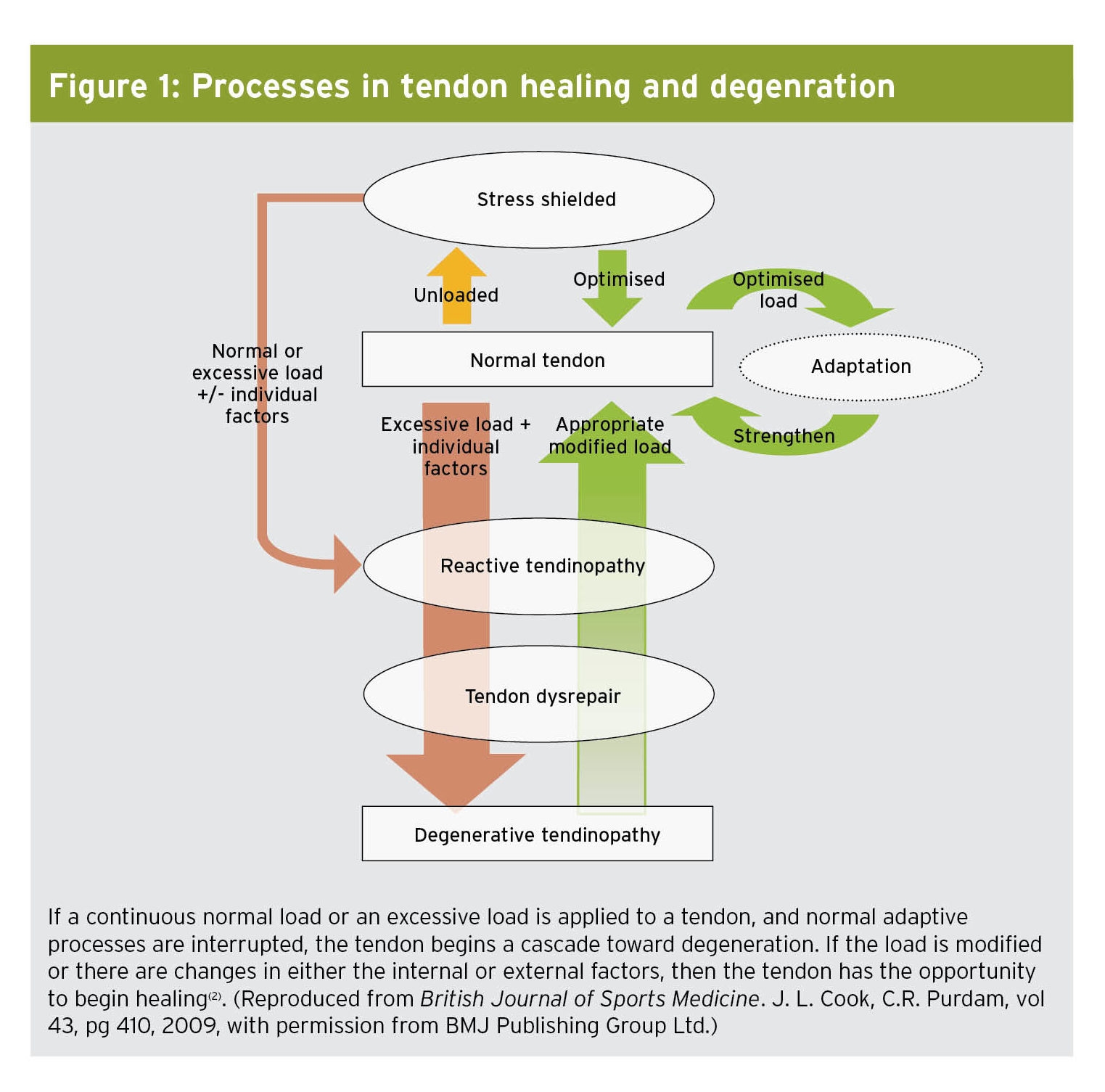
Figure 1: Processes in tendon healing and degeneration
Anti-inflammatory interventions
Since the inflammatory component of tendon healing occurs after the initial strain, it is reasonable to consider anti-inflammatory medications in the treatment of tendinopathy, especially in the period soon after injury. Corticosteroid injections provide short-term pain relief from pain and swelling. Some suppose that the effect is really on the tissues surrounding the injured tendon, rather than the tendon itself(3). Understanding that corticosteroid injections are not curative but palliative is important when considering their use in treatment. While improving comfort and function in the short term, corticosteroids tend to weaken the structure of the tendon, paradoxically increasing the risk of tendon rupture.
Nonsteroidal anti-inflammatory drugs (NSAIDs), administered either orally or trans-dermally, also assist with pain management. Professor Jill Cook has frequently stated that the use of Ibuprofen (and only Ibuprofen – not Celebrex or Feldene etc.) seems to drop the pain in painful tendons in the reactive stage if used short term(4). Again, some theorize that perhaps the mechanism of action is actually on the tissue surrounding the tendon. However, NSAIDs have been shown to suppress tendon healing in animal studies, which would indicate a direct action upon the tendon(3). Since inflammation is a normal part of the adaptive response, perhaps inhibiting it with NSAIDs interrupts a necessary part of the healing process.
The prolonged use of NSAIDs carries a risk of side effects related to the renal, cardiovascular and gastrointestinal systems and a review of the literature provides little to substantiate their use in the treatment of tendinopathy beyond short-term pain control(3). Therefore, the decision to recommend NSAIDs or not is based on clinical judgment. Consider the need for pain management versus the potential side effects and impact on tendon healing before advising its use.
Healing through loading
Eccentric exercise is the most effective type of exercise for treating tendinopathy, though the mechanism of action is not well understood(5). During eccentric exercise (muscle lengthening), the tension within the muscle fibers is greater than when the muscles move concentrically (muscle shortening), yet the energy requirements, oxygen consumption, adenosine triphosphate (ATP) breakdown, and heat production are all less than during concentric movement. Therefore, during eccentric exercise, the muscle produces the same amount of work with less effort and releases fewer irritating waste products.
Perhaps there is something in this eccentric equation that allows the tendon to receive the mechanical stimulation it needs to repair while keeping the biochemical irritants at bay. Using the Melbourne model of degenerative tendinopathy, it is reasonable to expect a less stressful load, along with the modification of intrinsic and extrinsic factors, to trigger a normal adaptive response(2). Studies conducted on tendinopathic Achilles and patellar tendons show good results using an eccentric training program(3).
Guidelines for treatment with eccentric loading typically rely on the Alfredson protocol. Alfredson and colleagues conducted a landmark Swedish study that showed heavy-load eccentric calf muscle exercise to be effective in the treatment of chronic Achilles tendinopathy(6). Since then, most studies recommend a similar regimen of three sets of 12-15 repetitions, first using body weight resistance and then progressing to a 15-repetition maximum resistance twice per day(3). Pain is tolerated during the exercise; however, if significant pain persists after the exercise, decrease the resistance. There are no contraindications to eccentric exercise.
Physiotherapy modalities
Physiotherapists and trainers often augment exercise prescriptions with various treatment modalities. While these may add to the subjective comfort of the athlete, they are not curative. For instance, cryotherapy (treating with ice) is a standard method of reducing inflammation and controlling pain. While effective for acute injuries, its efficacy in treating chronic tendinopathy in the reactive or disrepair stage is questionable. Other modalities, such as sound-assisted soft tissue massage, augmented soft tissue mobilization, ultrasound therapy, phonophoresis, and iontophoresis, lack randomized controlled clinical studies to support their use. These treatments are generally safe and have minimal side effects but may not promote tendon healing.
The question of blood flow
Increased blood flow in damaged tendons, seen in the tendon disrepair stage, was first observed and described in the 1990s and termed neovascularization(6). Nerves sprouting alongside new blood vessels were thought to be the cause of the pain in the diseased tendon. While some saw this increased vascularity as the body’s attempt to heal and sought ways to promote it, others concluded that preventing this angiogenesis would interrupt the failed healing cascade and also provide pain relief.
Shock therapy
Theoretically, the administration of shock waves to an injured tendon induces microtrauma that stimulates blood flow. Extracorporeal shock wave therapy (ESWT) targets tissue with a single-impulse acoustic wave generated using magnets, underwater electrodes, or crystals. Shock waves are uni-phasic and have a peak pressure that is nearly 1000 times greater than an ultrasound wave. Shock waves, administered as a low-energy treatment, require three weekly sessions; whereas, a high-energy treatment is a single session that requires local or general anesthesia.
Authors from the Queen Mary University of London recently reviewed the literature available on the use of ESWT to treat lower limb tendinopathy and found only 13 studies with enough reliable data to analyze(6). The protocol for the administration of ESWT varied between them, further complicating the ability to draw definite conclusions. That said, the researchers suggest that ESWT is more effective in the treatment of patellar tendinopathy than other conservative treatments and equal to patellar tendinopathy surgery. When treating insertional Achilles tendinopathy, they also suggest that ESWT is more effective than eccentric loading; and equally effective as eccentric loading when the tendinopathy is in the midportion of the Achilles tendon. There is some evidence that, when combined, ESWT and eccentric loading provide even better outcomes(7).
Go with the flow
There are several other treatments that aim to increase blood flow to the relatively avascular tendon in an attempt to improve healing. Topical glyceryl trinitrate, administered through a topical patch, delivers nitric oxide (NO) to the tendon through the skin. The NO is thought to improve collagen production and increase vascularization. Low-level laser therapy, also known as cold or soft laser, supposedly increases tendon metabolism, collagen production, and vascularisation.
Injections of platelet-rich plasma, derived from the subject’s own blood, directly into the tendon supposes that the platelets release growth factors into the damaged tissue (see Andrew Hamilton’s detailed article on platelet therapy). These growth factors purportedly decrease inflammation and stimulate revascularization and collagen production. Some have even attempted to inject whole autologous blood into diseased tendons with the same goals.
Overall, the shortage of studies on the efficacy of each of the above treatments and the variation in protocols, study design, and sample size makes it difficult to assess their usefulness with complete confidence. In addition, some of these treatments carry with them significant risks or side effects. Professional licensing boards and government agencies vary on approval of these methods, including ESWT, to manage tendinopathy; clinicians should, therefore, carefully assess the pros and cons of a treatment option before recommending its use for athletes.
Interrupting blood flow
While some treatments attempt to increase the blood flow to the damaged tendon, others try to stop it. In sclerotherapy, a chemical agent injected into the tendon causes thrombosis and deterioration of the newly formed vessels and nerves. Electrocoagulation uses a radiofrequency probe to transfer thermal energy to the diseased tendon and destroy the neovascular bundle. Injection with hypertonic glucose and lidocaine, called prolotherapy, works toward the same end while inducing an inflammatory response. The ability to test whether these treatments have the effect they intend is difficult. Studies showing their impact on function and pain are inconclusive(8).
Researchers from Qatar, Italy, and England questioned the validity of the theory of angiogenesis (new blood vessel growth) as a source of either healing or pain in a joint review of the literature on the subject(4). Conflicting evidence exists as to the presence of neovessels in symptomatic Achilles tendons. In fact, studies show neovascularisation in asymptomatic athletes and test subjects after strenuous exercise(4). Does the question arise as to whether the new vessels are part of a normal physiological response or a pathologic one?
The researchers concluded that the presence or absence of neovessels bears no significance on the diagnosis, prognosis, or magnitude of pain experienced by those with tendinopathy. Therefore, the treatments that attempt to influence neovasculature one way or the other, while initially promising, may prove to be ineffective with well-designed, randomized, and controlled research. Until research provides better conclusions, the recommendation of these treatments to athletes is not worth the potential risks associated with them.
Surgical options
Tendon surgery is generally considered a last resort for those who fail conservative treatment. Surgery removes the areas of the diseased tendon, along with the accompanying neovascularization, and stimulates a new healing process. This is accomplished through either ultrasound-guided percutaneous tenotomy, sometimes called needling, or tendon debridement and repair via arthroscopic or open procedures.
Success rates following surgery vary depending on the type of procedure, the tendon treated, and the level of injury within the tendon. Rehabilitation and return to sport can take upwards of 12 months, depending again on the type of procedure and severity of the injury. Most notably, 20% to 30% of surgical treatments fail to improve the symptoms(9).
Future treatments
Pioneered in veterinary medicine to treat horses, stem cell transplantation for tendinopathic tendons in humans is now under preliminary investigation. Autologous stem cells, derived from the subject’s own bone marrow, fat tissue, or tendon is either injected directly into the tendon or introduced into the tendon along with a scaffolding matrix that promotes the differentiation of the stem cells into tenocytes.
Another potential treatment is the injection of cultured tenocyte-like cells, taken from the subject’s own skin, into the injured tendon. These cells produce collagen and thus are thought to promote healing. Preliminary studies show promise, but placebo-controlled trials are needed to confirm efficacy.
Biomaterials, constructed from naturally occurring substances, have potential benefits in the treatment of tendinopathy. These biomaterials mimic native tendon structure and, therefore, drive cell production toward alignment as normal tendons. In the preliminary stages of the investigation, this type of tendon grafting has proved beneficial in canine subjects(10). Animal studies also show promise using the injection of growth factors directly into the tendon(10).
Recommendations for clinicians
Tendinopathy is among the most difficult sporting injuries to treat, and clinicians and athletes will grasp nearly anything with a promise to stop the pain and return the player to sport.
Anti-inflammatory medications, used immediately after injury or the onset of symptoms, reduce pain but possibly deter healing. Physiotherapy modalities may subjectively help with pain management but lack evidence that they are curative. Many other treatments, although theoretically sound, need more research and development and carry with them significant risks.
Modifying the load on the tendon through eccentric exercise and providing the tendon with sufficient rest through the alteration of contributing intrinsic or extrinsic factors gives the tendon the best opportunity to heal itself when in the reactive and disrepair stages (see box 2). The tendon may bear the load for a weak link in the kinetic chain, be overworked since starting twice-a-day practices, or become strained from abnormal positioning while sleeping (because the athlete’s dog insists on sleeping on the bed with its head on the athlete’s knee). Play detective until you find and remove the cause of strain. Only then will the tendon get the rest it needs and begin the healing process (see box 2).
You need to be logged in to continue reading.
Please register for limited access or take a 30-day risk-free trial of Sports Injury Bulletin to experience the full benefits of a subscription. TAKE A RISK-FREE TRIAL
TAKE A RISK-FREE TRIAL
Newsletter Sign Up
Subscriber Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Newsletter Sign Up
Coaches Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Be at the leading edge of sports injury management
Our international team of qualified experts (see above) spend hours poring over scores of technical journals and medical papers that even the most interested professionals don't have time to read.
For 17 years, we've helped hard-working physiotherapists and sports professionals like you, overwhelmed by the vast amount of new research, bring science to their treatment. Sports Injury Bulletin is the ideal resource for practitioners too busy to cull through all the monthly journals to find meaningful and applicable studies.
*includes 3 coaching manuals
Get Inspired
All the latest techniques and approaches
Sports Injury Bulletin brings together a worldwide panel of experts – including physiotherapists, doctors, researchers and sports scientists. Together we deliver everything you need to help your clients avoid – or recover as quickly as possible from – injuries.
We strip away the scientific jargon and deliver you easy-to-follow training exercises, nutrition tips, psychological strategies and recovery programmes and exercises in plain English.










